What Does a Subscript Indicate in a Chemical Formula
The lack of a number preceding the carbon symbol C and the compound formula CO2 shows that there is one carbon atom and one carbon dioxide molecule. Subscripts in Chemical Formulas Subscripts are important numbers in formulas especially when youre trying to understand how many atoms of an element are present.
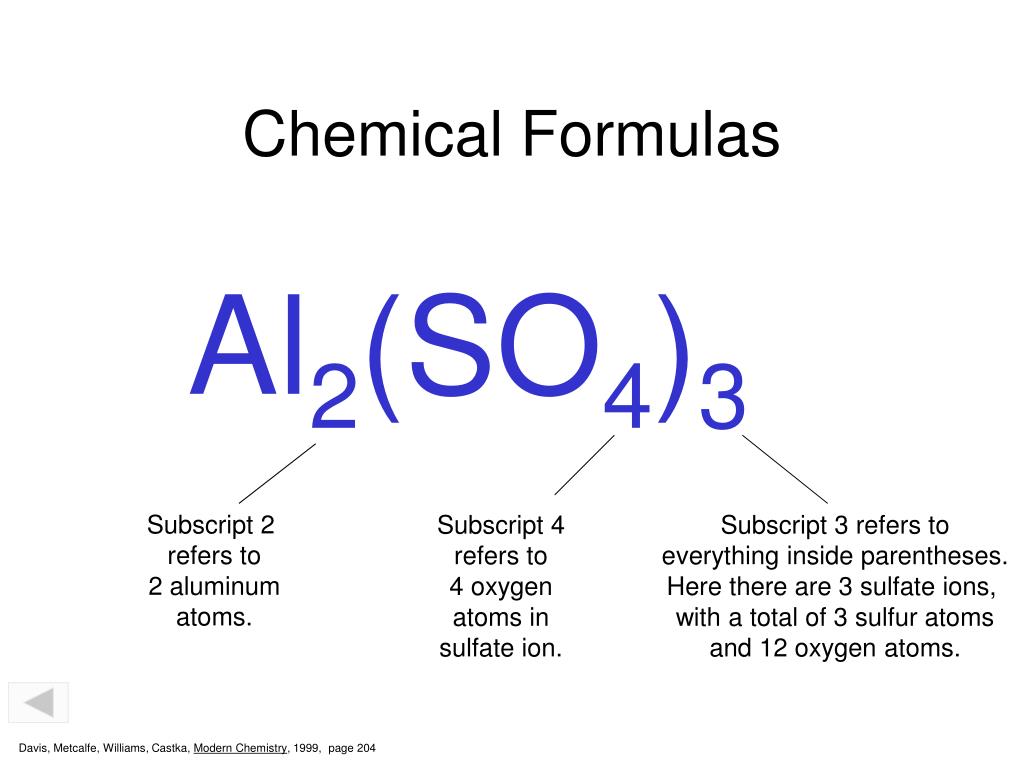
Ppt Subscripts Superscripts And Coefficients Powerpoint Presentation Id 4272565
The subscripts for the chemical.

. The numbers appearing as subscripts in the chemical formula indicate the number of atoms of the element immediately before the subscript. This shows that there are three times the number of atoms which are in the brackets. A chemical formula identifies each constituent element by its chemical symbol and indicates the proportionate number of atoms of each element.
In a chemical formula a subscript is a number written to the right and slightly below the symbol for the chemical element. If the subscript is 1 it is not written. Subscripts that are small figures towards the lower right of the symbol are utilized to represent the amount of ions of every aspect in an ionic compound.
Molecular formulas do not indicate how the atoms are arranged in the molecule. In these materials the atoms are not necessarily covalently bonded to eachother and this allows for the non whole number integers. Answer In chemistry a subscript is used to indicate the number of atoms of an element in a molecule.
Here each element is represented by its atomic symbol obtained from the periodic table followed by a subscript. What does the subscript represent in a chemical formula. In a molecular formula a subscript is written immediately after the letter denoting an element and refers to the amount of atoms that element has.
The subscript lower represents the number of each atom while the superscript higher. You might be interested in. In a chemical formula the letters represent the atomic symbol of each atom.
Subscript numbers in chemical formulas represent the number of atoms or molecules immediately preceding the subscript. If no subscript seems one atom of this element exists. If for example they weighed out 1 g of material and performed elemental analysis for each element then the subscripts you see would be the ratio of that element.
The empirical formula tells the lowest w. Stoichiometry is the process of balancing chemical equations and subscripts in chemical formulas play in important role. Each letter in a formula symbolizes one element from the.
In empirical formulae these proportions begin with a key element and then assign numbers of atoms of the ot. The constituent elements and atoms of any species in chemistry are expressed in the form of a chemical formula. What does the subscript number indicate in a chemical formula.
Jun 1 2014. It indicates that there are a certain number of atoms in a compound. If no subscript appears one atom of that element is present.
The subscript in a chemical formula represents the amount of that atom in that compounds formula. O - 4 x 3 12 atoms. A subscript is a number to the right and below the abbreviation of an element that indicates the number of elements.
What Does The Chemical Formula Of A Compound IndicateA chemical formula is an expression that shows the elements in a compound and the relative proportions of those elements. A molecular formula or empirical formula is noted as a series of letters and numbers that represent a compound or a molecule. The figures appearing as subscripts within the chemical formula indicate the amount of atoms from the element immediately prior to the subscript.
Meaning of Subscript Numbers. S - 1 x 3 3 atoms.

Molecular Formula Ck 12 Foundation

Do Now 1 What Is A Chemical Formula Give An Example 2 What Is A Chemical Equation 3 What Does The Law Of Conservation Of Mass Matter Say Agendatitle Ppt Download

0 Response to "What Does a Subscript Indicate in a Chemical Formula"
Post a Comment